Conference trips can bring new collaborations, especially when you are open to discussions and meeting new people (read scientists). At the 2nd French-Polish Chemistry Congress held in 2023, Martin heard Magdalena Rowińska-Żyrek give a talk on the antimicrobial properties of Zn- and Cu-complexes with peptides from shrimp and this meeting sparked cooperation between our group and the group from Wroclaw which just resulted in a paper published in Chemical Science1.
Since Magda (Wiloch), who studies the redox activity of Cu(II) complexes with β-amyloid peptides in our group, had shown that these can promote the production of reactive oxygen species in the body, the idea that the shrimp peptides might do the same, and this might be a contributing factor to their antimicrobial activity was compelling. Magda W was eager to start examining the electrochemical properties of this new interesting peptide derived from shrimp – PvHCt.
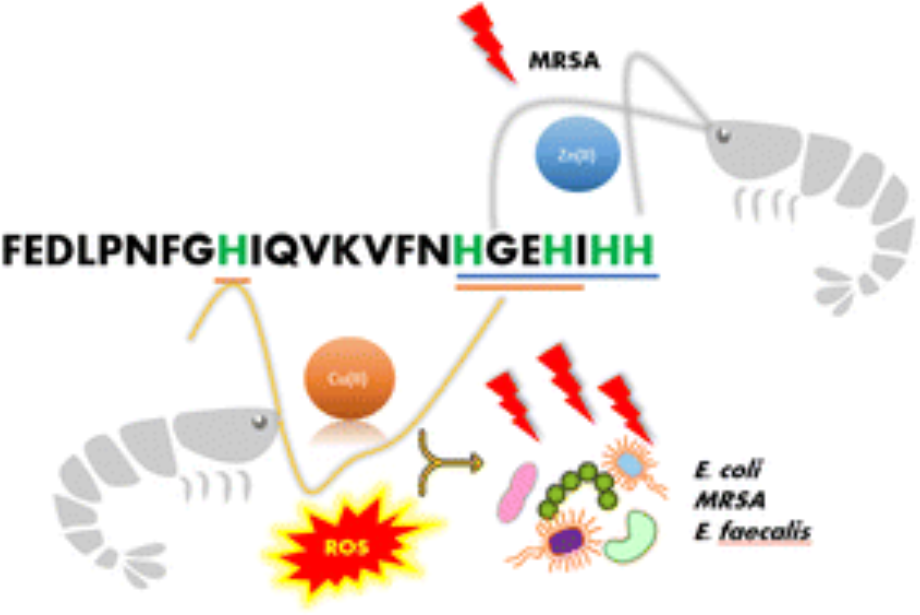
The paper is a collaboration between several groups, and using NMR, UV-vis, CD and FTIR spectroscopies, along with cyclic voltammetry, potentiometry, and DFT calculations, it could be demonstrated that Cu(II) binds to PvHCt, a 23-amino acid long histidine-rich peptide. Based on the experiments, it was found that the creation of Cu(II)-PvHCt complex increased the α-helical content and the production of reactive oxygen species (ROS), all of which contribute to the remarkable antimicrobial potency of PvHCt.
Voltammetric studies allowed us to investigate redox reactions of Cu(II) ions bound by PvHCt. CVs recorded for the Cu(II)-PvHCt complex at lower potentials revealed an irreversible Cu(II)/(I) redox process with a slow electron transfer. This reaction could be connected with the formation of harmful ROS under biologically relevant conditions because of the formal potential close to 0V. Thus, electrochemical studies have shown why PvHCt peptide exhibits antimicrobial properties after binding Cu(II) ions.
This research shows clearly that there is a significant and underestimated effect of metal coordination on the activity of antimicrobial peptides. As far as we can tell, this is the first work which describes a Cu(II)-induced structural rearrangement of an antimicrobial peptide, which leads to increased ROS production and triggers its antimicrobial potency.
- A. Miller, A. Matera-Witkiewicz, A. Mikołajczyk, A. Kola, M. Z. Wiloch, M. Jönsson-Niedziółka, R. Wieczorek, J. Wątły, D. Valensin, M. Rowinska-Zyrek
Cu(II) binding to an antimicrobial shrimp peptide – a small step for structural chemistry, a big leap for medicinal applications, Chem. Sci. (accepted) (link – OA)