In the latest round of announcements from NCN we were delighted that Martin’s Opus application for creating new biosensors based on peptides was selected for financing. This project was written and will be executed in collaboration with Dr Katarzyna Szot-Karpińska from our neighbours in the Surface Nanoengineering Group. The scientific goal of this project is to push the limits of detections for point-of-care sensors by using an electrowetting-based system for sample mixing to enhance receptor-analyte binding kinetics, and phage display technology to isolate high affinity peptide-based receptors. The result will hopefully be a point-of-care testing sensor for measurements directly in whole blood of biomarkers of neurodegenerative diseases based on peptide recognition elements.
This project is a spiritual follow-up to our work on CRP-based biosensors started by Kasia and Meen, and was published in ACS Sensors, Sensors and Actuators, and Analytical Chemistry 1.
Applications to NCN must include a popular science description. We tried to explain the importance of our biosensors project invoking Elizabeth Holmes, COVID-19 and the Nobel Prize:
During the COVID-19 pandemic, the development of accurate tests that could quickly tell if a person was infected or not was a game-changer in limiting the spread of the disease. But the dream of quickly and cheaply diagnosing a wide range of diseases from just a few drops of biological fluids such as blood or saliva didn’t start there. It was this promise that made Elizabeth Holmes, founder of the startup Theranos, a billionaire and media darling after convincing investors that her company had developed a healthcare device that would revolutionise medical diagnostics. While Theranos was built on a lie, and Holmes is now in jail, technology is getting to the point where some of that promise is coming true.
For a number of diseases, specific biomarkers – molecules associated with that particular condition – have been identified, such as Prostate Specific Antigen for prostate cancer and ERBB2 for many other aggressive forms of cancer. Other biomarkers that have become increasingly interesting over the last years are those that are indicative of neurodegenerative diseases like dementia, Alzheimer’s or Parkinson’s. Here, regular monitoring of biomarker levels could reveal these conditions before symptoms appear, which increases the probability of slowing their progress.
Devices such as the COVID-tests or pregnancy tests that can be used outside of lab settings are often referred to as point-of-care sensors. While these mentioned sensors only give a simple yes or no, for other purposes a numerical value is needed. This is the case for the biomarkers for neurodegenerative diseases or cancer, where the change in levels of biomarkers over time can indicate problems.
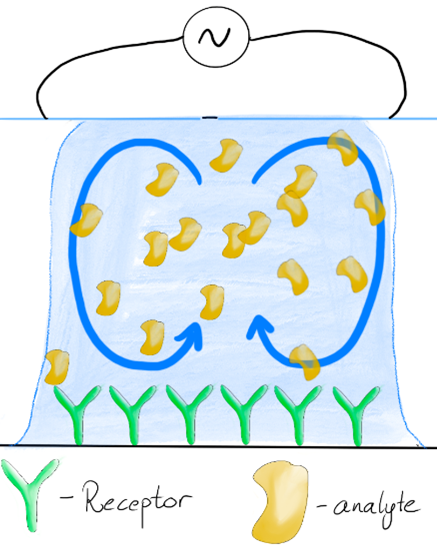
In this project, we will develop electrochemical point-of-care sensors for neurodegenerative diseases. This will be a novel type of sensor where the molecule that recognises the biomarker is a peptide instead of the commonly used antibodies. We will also spend a lot of effort making sure the sensor is low-cost and easy to use so that it might be used by a large number of people without the need to await results from a laboratory test. For this purpose, we will work with a passive flow system, and to shorten the measurement time, we will use an electrowetting-based system for stirring the sample droplet to ensure that the biomarkers are efficiently getting into contact with the recognition peptide.
However, the biggest challenge will be to make these sensors both simple to use and sufficiently sensitive to measure the very small amounts of biomarkers that circulate with the blood. Therefore, we will use a technique known as phage display, which was recently awarded with the Nobel Prize, to identify new peptides with very high affinity to attach to the biomarker we have selected to study.