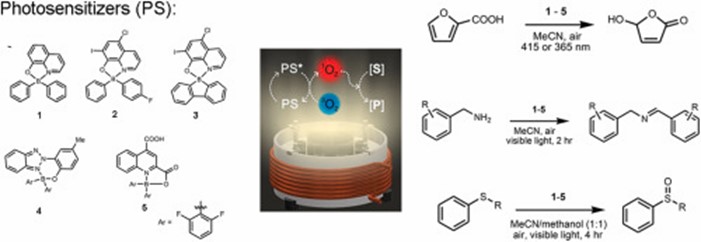
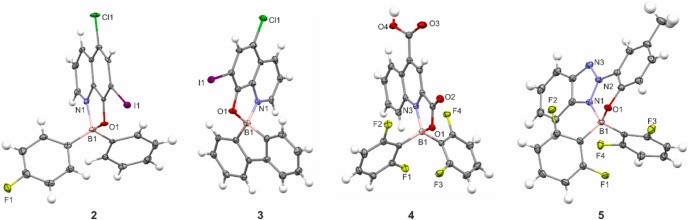
Magda once again cooperated with the Warsaw University of Technology (prof. Tomasz Kliś). As part of this cooperation, a new article entitled was published in Dyes and Pigments1. The group from WUT obtained 5 boron-containing dyes. These substances were tested as photosensitizers for singlet oxygen activation. Among the studied compounds, the diarylborinic derivative bearing a 5-chloro-8-oxy-7-iodoquinolinate ligand was selected as the most economical singlet oxygen sensitizer. Magda’s role was to conduct electrochemical studies to determine the redox properties of the compounds. Based on cyclic voltammetry and spectroscopic data the excited-state redox potentials were calculated (good news, results are found to be in good agreement). The obtained potentials indicate that among the studied compounds 4 is the most powerful excited–state oxidant, whereas 5 is the most powerful excited–state reductant.
We are tremendously pleased with the cooperation and our shared success!
- T. Kliś, A. Blacha-Grzechnik, K. Durka, K. Mazurek, A. Szymańska, M. Z. Wiloch, M. Ziółkowska
Photocatalytic oxidation of benzylamines and sulfides using selected organoboron complexes as molecular oxygen activators, Dyes Pigm. 231, 112371 (2024). (link)