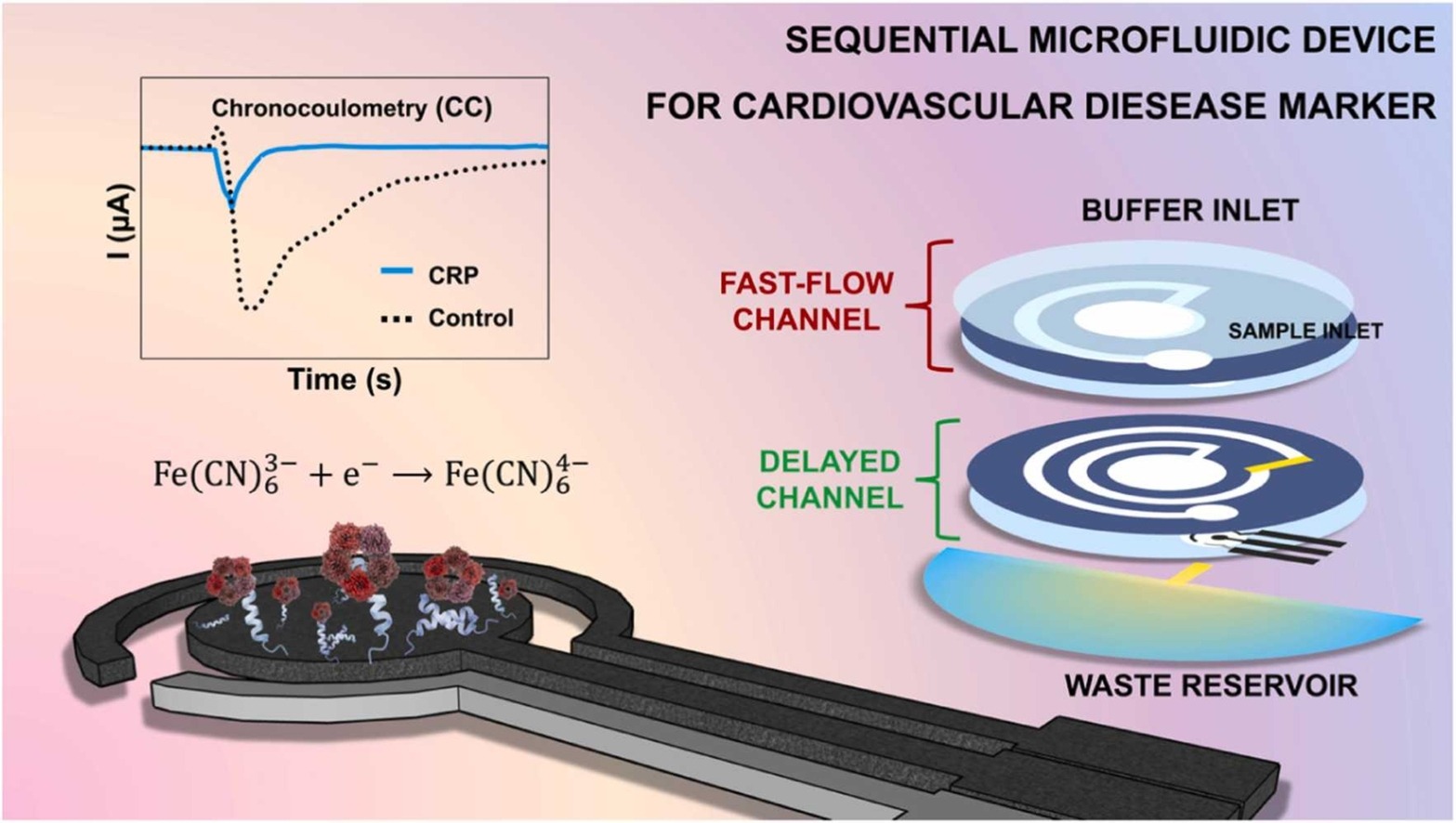
Measuring CRP levels in blood usually requires sending a blood sample to the lab for an ELISA test. This takes time… the results typically come only the next day. It would be so much more convenient if you could get the result directly in your doctor’s office.
In our latest paper1 we created a microfluidic device that enables measurements of CRP concentration directly in blood in less than 15 minutes. We also took care to ensure that the measurements can be performed with only minimal user intervention. Two steps are needed. First the sample is loaded and a waiting time is needed for the binding to occur between the CRP in the sample and the biorecognition elements on the electrode surface. Later, a buffer solution is added that flows through two channels; the fast channel for washing away the sample and unbonded target molecules, and the delayed channel through which a redox probe is flowing across the electrode. Depending on the CRP concentration, we will see a different amount of blocking of the electrode, and thus a lower current. This can then be used to calculate the original CRP concentration in the sample.
For this study, we used a peptide found in a bacteriophage as recognition element (already presented in this paper). We show that by varying the peptide concentration on the electrode surface we can tweak the sensitivity of the electrode to cover different concentration ranges.
This work was mainly done by our postdoc, Meen (Suchanat) in collaboration with our neighbours from the Surface Nanoengineering group.
- S. Boonkaew, K. Szot-Karpińska, J. Niedziółka-Jönsson, B. Pałys, M. Jönsson-Niedziółka
Point-of-care testing for C-reactive protein in a sequential microfluidic device, Sens. Actuators B Chem. 397, 134659 (link – OA)


5 thoughts on “Point-of-care testing for C-reactive protein in a sequential microfluidic device”
Using point-of-care testing for C-reactive protein in a microfluidic device sounds fascinating! I’d love to know more about how this approach improves diagnostic speed and accuracy. Exciting work!
The main effect on accuracy is the application of the novel recognition element. Both here, where we use a peptide, and in our follow-up work with nanobodies, the smaller molecule gives a better signal than antibodies. This is likely related to the size of the molecule, which affects both the density on the surface and the difference between the size of the recognition element itself and its complex with the CRP.
As for speed, we sacrifice some of the sensitivity for speed when we decide not to wait for the signal to saturate before measuring.