Pillararenes were introduced as a novel class of macrocyclig host molecules in 2008 when 1,4-dimethoxypillar[5]arene (P5A) was synthesised. The pillararenes are similar in structure to the better known cyclodextrins or calixarenes and they can also form host-guest complexes with smallish molecules that can fit into their cavities.
In a recent paper1 we investigated the electrooxidation of P5A in three common aprotic solvents (acetonitril, dichloroethane and dichloromethane). Our original plan was to use pillararenes as a kind “ionophore” in liquid-liquid electrochemistry to selectively induce a guest-molecule to transfer from the aqueous to the organic phase. As a part of that work, we measured the redox properties of the pillararenes themselves in the organic solvent.
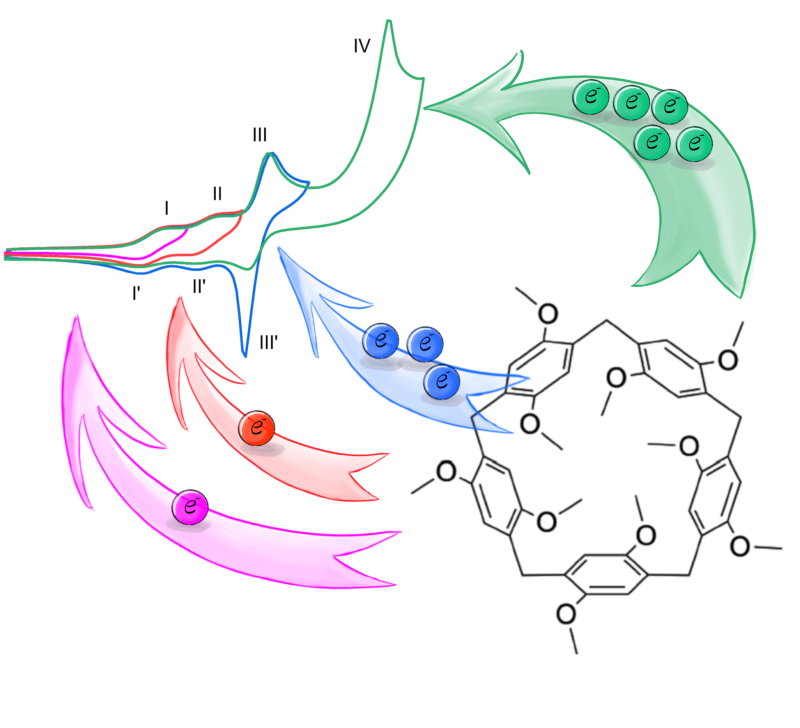
We noticed that P5A in DCM gives three (quasi-)reversible oxidation peaks, with different heights. If scanned to even more positive potentials a large irreversible peak appears. We simulated the spectra in Comsol and managed to get a very good fit to the data, showing that the number of electrons for the two first peaks is one, and that three electrons are involved in the third oxidation. The associated reduction peak is very sharp, indicating that the P5A<sup>5+</sup> is less soluble and adsorbs on the electrode surface. The large irreversible oxidation peak involves an additional five electrons, and spectroelectrochemical measurements confirmed that this transforms the arenes to their respective quinones.
The measurements is all three solvents were quite similar, all showing the same four peaks. However, in ACN, the peaks are less well-formed and it was not possible to get a good fit to the data in the simulations. This means the reactions do not follow simple Butler-Volmer kinetics, something that we attribute to interactions between the P5A and the solvent molecules.
During the course of the experiments a paper2 appeared with the same measurements as ours for one of the solvents. Their results were very similar to ours, but the electron numbers they assigned are different to ours. We think that out paper is still a useful addition to the knowledge about these new macrocyclic molecules, even if we were slightly scooped.
As for the liquid-liquid measurements; we never managed to get them to work.
The paper is published in a special issue of ChemElectroChem to celebrate the 65th birthday of Marcin Opallo, the director of our institute and the former postdoc supervisor for Martin.
- M. Z. Wiloch, E. Kuna, S. Kosiorek, V. Sashuk, M. Jonsson-Niedziolka
The spectroelectrochemical behaviour of 1,4‐dimethoxypillar[5]arene (P5A) and its monomer in different organic solvents, ChemElectroChem accepted (2021). (link) - N. Pearce, E. S. Davies, N. R. Champness, Molecules, 25, 1627 (2020).