After a long haul, our paper describing three-phase experiments with a new hydrophobic Ru-complex redox-probe was finally published in Electrochimica Acta1. This work is a collaboration between our group and Prof. Masa-Aki Haga’s at Chuo University in Tokyo. About two years ago Haga visited our institute and gave a talk, among other things, about the ruthenium complexes his group synthesises that can be used for photochemistry, but that are also oxidisable and quite hydrophobic. We thought that these could be useful for our liquid-liquid experiment. The problem was that the molecules Haga presented were all ionic. So we managed to gather some funds from our institute and from Chuo University to send Vishwanath to Japan to synthesise a neutral Ru-complex that we could use.
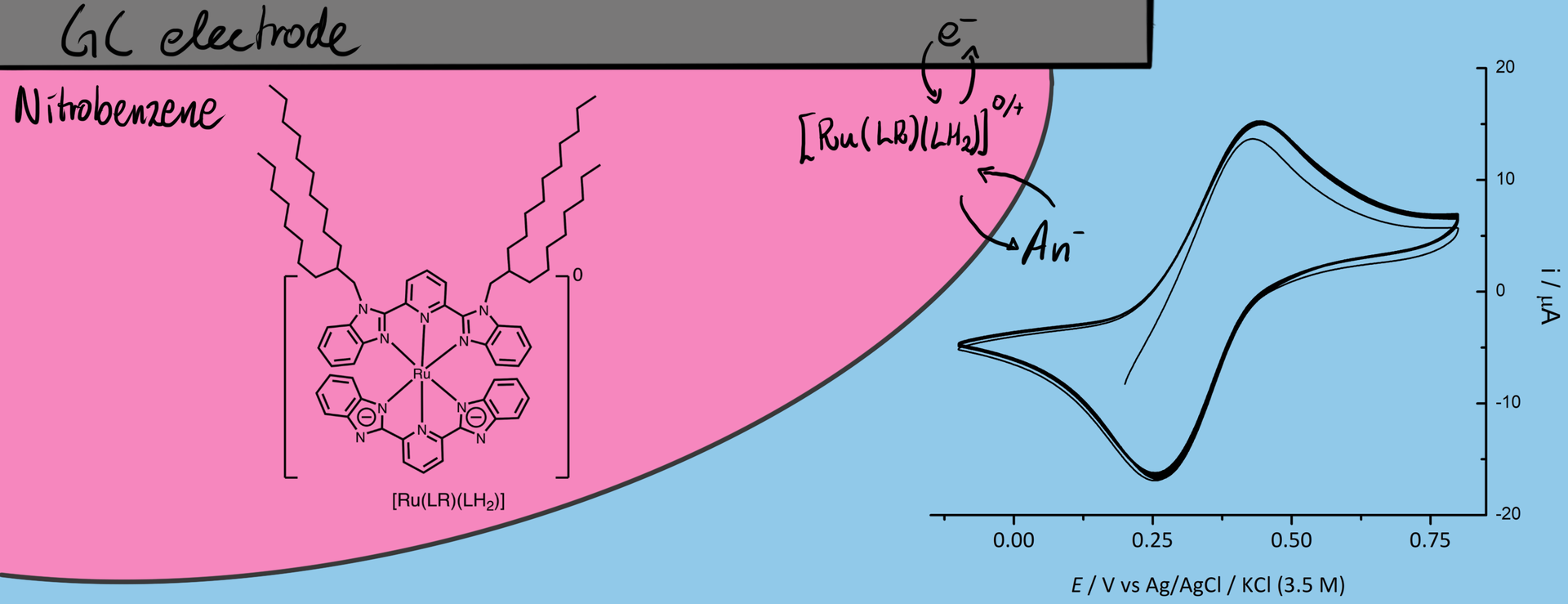
Finally in February last year, Vishwa left for two months to Haga’s lab in Tokyo and managed to synthesise and characterise a useful compound. He did some of the electrochemical measurements already in Tokyo, and these showed that the compound had nice reversible oxidation of the Ru(II) to Ru(III) that was associated with anion transfer at the three-phase junction.
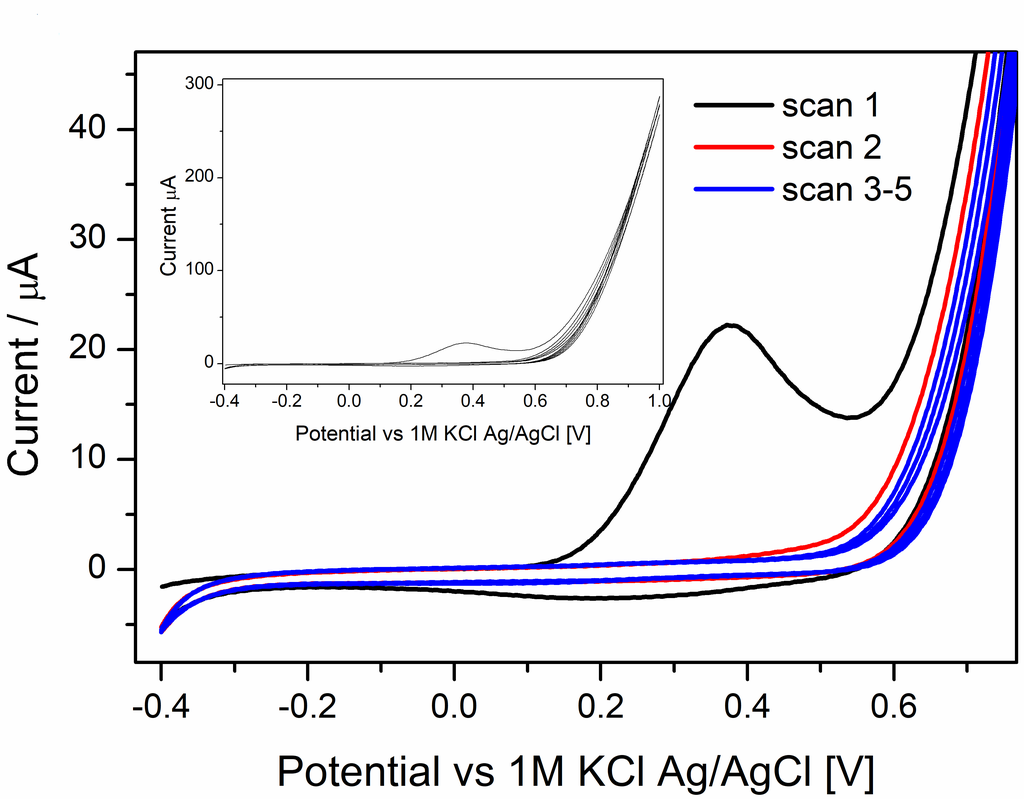
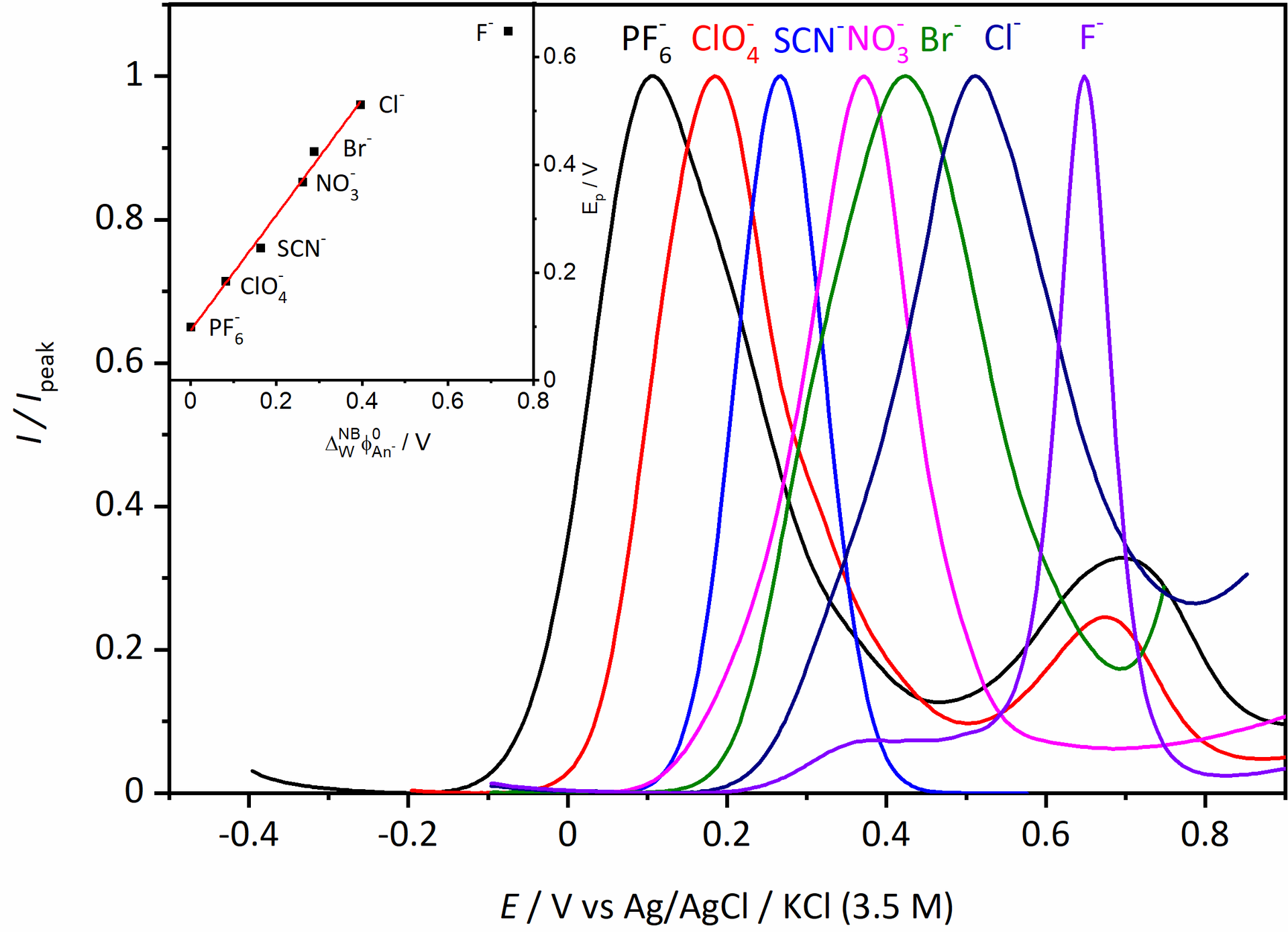
However, we saw additional features in the voltammogram involving the ion PF6–, ClO4–, and SCN– that required additional experiments to figure what was going on. For the two former ions an additional peak showed up at around 0.7 V, which we didn’t manage to explain despite a large number of additional measurements. For thiocyanide, the CV became completely irreversible if scanned to too high potentials. We attribute that to the interaction with the strongly coordinating anion. The large increase in current at high potential, suggests a different stoichiometry than the oxidation of the [RuII(LR)(L)]0 complex. Maybe this could be some catalytic water oxidation? Or something else completely. It might be interesting to investigate, but that would require synthesis of more of the Ru-compound for the experiments.
We had thought that this manuscript could be of interest to a wider chemical audience, so it was first submitted to a general chemistry journal but as we never made it past the editors we eventually ended up publishing it in Electrochimia Acta, which is a very good electrochemistry journal. However, a pre-print has been available on ChemRxiv since April where it has been downloaded more than 200 times.
- Vishwanath R.S, M. Haga, T. Watanabe, E. Witkowska Nery, M. Jönsson-Niedziolka
Three-Phase Electrochemistry of a Highly Lipophilic Neutral Ru-Complex Having Tridentate Bis(benzimidazolate)pyridine Ligands, Electrochim Acta, Accepted (link)(ChemRxiv Preprint)