Corrosion is a part of electrochemistry that our group is generally not involved in, although it is a very important area of research. However, Martin again had a small part in a recently published paper*, this time about corrosion protection. Specifically the paper is examining the properties of zinc coatings on steel that have been passivated using sodium molybdate.
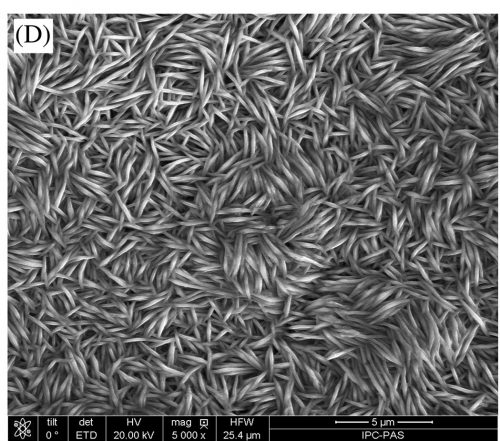
To protect iron and steel products from corrosion, zinc coatings because it is quite effective and also due to its low cost of application on carbon steel. However, exposed to humid air and industrial gases, zinc can corrode and white‐gray corrosion products are formed on the its surface. To increase the corrosion resistance of the coating zinc is passivated, creating additional protective films on the surface.
For a long time solutions of hexavalent chromium salts were used for passivation resulting in conversion films on zinc with highly protective properties. Unfortunately Cr(VI) is toxic and harmful to the environment, so it was banned by the European Union banned for the treatment of parts used in the automotive industry in 2000 and in 2011 a directive was adopted to limit the use of hazardous and harmful substances in electrical and electronic equipment.
In this paper a chromium free treatment based on sodium molybdate is used to passivate zinc coatings and the properties of the films are studied. Measurements using SEM, EDX, XPS, surface profilometry and electrochemical impedance spectroscopy were performed on films electrodeposited using different times and different current densities. The different films had different surface structure and corrosion resistance, but the conclusion was that the if applied properly electrochemical passivation of zinc in a solution of sodium molybdate can be considered as a replacement for chromate conversion coatings.
* N. Akulich, N. Ivanova, I. Zharskii, M. Jönsson‐Niedziółka; Properties of zinc coatings electrochemically passivated in sodium molybdate. Surf. Interf. Anal. 50, 1310-1318 (2018).